Abstract of Publication No. 484
 Colette Boskovic, Andreas Sieber, Grégory Chaboussant, Hans U. Güdel, Jürgen Ensling, Wolfgang Wernsdorfer,
Antonia Neels, Gael Labat, Helen Stoeckli-Evans and Stefan Janssen
Colette Boskovic, Andreas Sieber, Grégory Chaboussant, Hans U. Güdel, Jürgen Ensling, Wolfgang Wernsdorfer,
Antonia Neels, Gael Labat, Helen Stoeckli-Evans and Stefan Janssen
Synthesis and Characterization of a New Family of Bi-, Tri-, Tetra-, and Pentanuclear Ferric Complexes
Inorg. Chem. 43, 5053-5068 (2004)
![]()
![]()
![]()
![]()
Abstract:
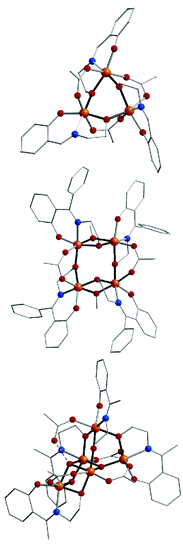
Nine members of a new family of polynuclear ferric complexes have been
synthesized and characterized. The reaction of Fe(O2CMe)2
with polydentate Schiff base proligands (H2L) derived from
salicylidene-2-ethanolamine, followed in some cases by reaction with carboxylic
acids, has afforded new complexes of general formulas
[Fe2(pic)2(L)2] (where pic– is the
anion of 2-picolinic acid),
[Fe3(O2CMe)3(L)3],
[Fe4(OR)2(O2CMe)2(L)4],
and [Fe5O(OH)(O2CR)4(L)4]. The tri-,
tetra-, and pentanuclear complexes all possess unusual structures and novel core
topologies. Mössbauer spectroscopy confirms the presence of high-spin ferric
centers in the tri- and pentanuclear complexes. Variable-temperature magnetic
measurements suggest spin ground states of S = 0, 1/2, 0, and 5/2 for the
bi-, tri-, tetra-, and pentanuclear complexes, respectively. Fits of the
magnetic susceptibility data have provided the magnitude of the exclusively
antiferromagnetic exchange interactions. In addition, an easy-axis-type magnetic
anisotropy has been observed for the pentanuclear complexes, with D
values of approximately -0.4 cm–1 determined from modeling
the low-temperature magnetization data. A low-temperature micro-SQUID study of
one of the pentanuclear complexes reveals magnetization hysteresis at nonzero
field. This is attributed to an anisotropy-induced energy barrier to
magnetization reversal that is of molecular origin. Finally, an inelastic
neutron scattering study of one of the trinuclear complexes has revealed that
the magnetic behavior arises from two distinct species.