Abstract of Publication No. 512
 Curtis P. Berlinguette, Alina Dragulescu-Andrasi, Andreas Sieber, Hans-Ulrich Güdel,
Catalina Achim and Kim R. Dunbar
Curtis P. Berlinguette, Alina Dragulescu-Andrasi, Andreas Sieber, Hans-Ulrich Güdel,
Catalina Achim and Kim R. Dunbar
A Charge-Transfer-Induced Spin Transition in a Discrete Complex:
The Role of Extrinsic Factors in Stabilizing Three Electronic Isomeric Forms of a Cyanide-Bridged
Co/Fe Cluster
J. Am. Chem. Soc. 127, 6766-6779 (2005)
![]()
![]()
![]()
![]()
Abstract:
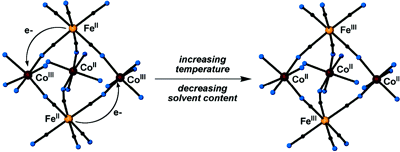
A series of bimetallic, trigonal bipyramidal clusters of type
{[Co(N-N)2]3[Fe(CN)6]2} are
reported. The reaction of {Co(tmphen)2}2+ with
[Fe(CN)6]3– in MeCN affords
{[Co(tmphen)2]3[Fe(CN)6]2}
(1). The cluster can exist in three different solid-state phases: a red
crystalline phase, a blue solid phase obtained by exposure of the red crystals
to moisture, and a red solid phase obtained by desolvation of the blue solid
phase in vacuo. The properties of cluster 1 are extremely sensitive to
both temperature and solvent content in each of these phases.
Variable-temperature X-ray crystallography; 57Fe Mössbauer,
vibrational, and optical spectroscopies; and magnetochemical studies were used
to study the three phases of 1 and related compounds,
Na{[Co(tmphen)2]3[Fe(CN)6]2}(ClO4)2 (2),
{[Co(bpy)2]3[Fe(CN)6]2}[Fe(CN)6]1/3 (3), and
{[Ni(tmphen)2]3[Fe(CN)6]2}
(4). The combined structural and spectroscopic investigation of
1-4 leads to the unambiguous conclusion that 1 can
exist in different electronic isomeric forms,
{CoIII2CoIIFeII2} (1A),
{CoIIICoII2FeIIIFeII}
(1B), and {CoII3FeIII2}
(1C), and that it can undergo a charge-transfer-induced spin transition
(CTIST). This is the first time that such a phenomenon has been observed for a
Co/Fe molecule.