Abstract of Publication No. 308
 M. Atanasov, T. C. Brunold, H. U. Güdel and C. Daul
M. Atanasov, T. C. Brunold, H. U. Güdel and C. Daul
Charge-Transfer Spectra and Bonding in Tetrahedral MnVI,
CrV, and VIV
and MnVII, CrVI, and VV Oxo Anions
Inorg. Chem. 37, 4589-4602 (1998)
![]()
![]()
Abstract:
Density functional theory (DFT) calculations on the tetrahedral MnVI,
CrV, and VIV(d1) oxo anions in their ground and
lowest excited d-d and O ![]() M charge transfer
(CT) states are reported and used to assign the electronic absorption spectra by
reference to the spectra of the isoelectronic MnVII, CrVI,
and VV (d0) and the MnV and CrIV
(d2) anions. Calculated geometrical shifts along the totally
symmetric metal-ligand vibration (a1)
for electronic excitations are in agreement with data
deduced from experimental vibronic fine structures, supporting the proposed
assignments. Using a CT model including (as different from DFT) configuration
interaction (CICT), it is shown that the CT excited states of
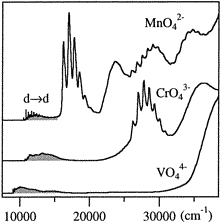
MnO42- at 17000, 23300, and 28200
cm–1 are due to d2
3A2(2e2), 1E(2e2), and
3A2(2e2) final states combining with a single
hole (L) on the ligand 1t1 and 4t2 orbitals,
respectively. The higher 10Dq and smaller B values for the
d2L(d1) states compared to those of the
d2 systems correlate with the shortening of the metal-ligand bond
accompanying the removal of electrons from the antibonding d orbitals, leading
to an increase in covalency and a change in the ordering of CT states for
CrV with
3T2(2e15t21)L
(10Dq) at a higher energy than 1E(2e2)L
(8B + 2C) as compared to CrIV with nearly degenerate
3T2(2e15t21) and
1E(2e2) terms. This allows one to estimate the energy of
the 3A2(2e2)L
M charge transfer
(CT) states are reported and used to assign the electronic absorption spectra by
reference to the spectra of the isoelectronic MnVII, CrVI,
and VV (d0) and the MnV and CrIV
(d2) anions. Calculated geometrical shifts along the totally
symmetric metal-ligand vibration (a1)
for electronic excitations are in agreement with data
deduced from experimental vibronic fine structures, supporting the proposed
assignments. Using a CT model including (as different from DFT) configuration
interaction (CICT), it is shown that the CT excited states of
MnO42- at 17000, 23300, and 28200
cm–1 are due to d2
3A2(2e2), 1E(2e2), and
3A2(2e2) final states combining with a single
hole (L) on the ligand 1t1 and 4t2 orbitals,
respectively. The higher 10Dq and smaller B values for the
d2L(d1) states compared to those of the
d2 systems correlate with the shortening of the metal-ligand bond
accompanying the removal of electrons from the antibonding d orbitals, leading
to an increase in covalency and a change in the ordering of CT states for
CrV with
3T2(2e15t21)L
(10Dq) at a higher energy than 1E(2e2)L
(8B + 2C) as compared to CrIV with nearly degenerate
3T2(2e15t21) and
1E(2e2) terms. This allows one to estimate the energy of
the 3A2(2e2)L
![]() 1E(2e2)L
transition from the CT (d2L) spectrum of
CrV(d1), which could not be observed for CrIV.
From a comparison of calculated and experimental oscillator strengths and
Huang-Rhys factors (S) for the lowest CT band in the VV,
CrVI, and MnVII (d0) and the VIV,
CrV, and MnVI (d1) oxo anions, it is shown that
the increase in covalency from left to right in this series is accompanied by a
reduction in band intensity and S for the progression in the
a1 vibration. An explanation
of this result in terms of ionic contributions to the metal-ligand bond
increasing from MnVI to CrV and VIV is
proposed. Intensities of "d-d" transitions display the opposite trend;
increasing covalency leads to stronger mixing between d
1E(2e2)L
transition from the CT (d2L) spectrum of
CrV(d1), which could not be observed for CrIV.
From a comparison of calculated and experimental oscillator strengths and
Huang-Rhys factors (S) for the lowest CT band in the VV,
CrVI, and MnVII (d0) and the VIV,
CrV, and MnVI (d1) oxo anions, it is shown that
the increase in covalency from left to right in this series is accompanied by a
reduction in band intensity and S for the progression in the
a1 vibration. An explanation
of this result in terms of ionic contributions to the metal-ligand bond
increasing from MnVI to CrV and VIV is
proposed. Intensities of "d-d" transitions display the opposite trend;
increasing covalency leads to stronger mixing between d
![]() d and CT excited states and thus an increase in
intensity.
d and CT excited states and thus an increase in
intensity.