Abstract of Publication No. 321
 Stefan R. Lüthi, Hans U. Güdel and Markus P. Hehlen
Stefan R. Lüthi, Hans U. Güdel and Markus P. Hehlen
Influence of the chemical environment on the electronic
structure and spectroscopic properties of Er3+ doped
Cs3Lu2Cl9,
Cs3Lu2Br9, and
Cs3Y2I9
J. Chem. Phys. 110, 12033-12043 (1999)
![]()
![]()
Abstract:
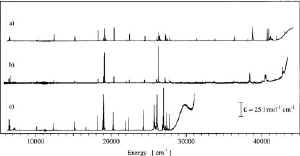
Energies and intensities of 114, 101, and 76 f-f
absorption transitions of Er3+ are determined by
high-resolution spectroscopy in the closely related host lattices
Cs3Lu2Cl9,
Cs3Lu2Br9, and
Cs3Y2I9, respectively. The observed
trends in the energy-level structure reflect the increasing covalency
and the length of the Er3+–X– bond. The
decreasing Coulomb repulsion of the 4f electrons, spin-orbit
coupling, and crystal-field potential reduces the energy splittings
of the SL, SLJ, and SLJMJ
states by 0.5%, 0.5%, and 25%, respectively, along the series
Cl–Br–I. Energy-level calculations that include crystal-field and
correlation crystal-field terms in the effective Hamiltonian,
reproduce most of the experimentally found trends. Root-mean-square
standard deviations of 18.0, 19.2, and 21.9 cm–1 are
reached in least-squares fits to the experimental crystal-field energies.
The f-f transition intensities increase along the
series Cl–Br–I as a result of the decreasing energy of the
f-d bands. In the iodide compound, where the first
f-d bands are as low as 30000 cm–1, this
influence is especially pronounced for the f-f
absorptions at higher energy. The quality of the wavefunctions
obtained in the energy-level calculations is not sufficient to
reliably calculate the relative absorption intensities of individual
crystal-field components within a given multiplet transition. This
deficiency is ascribed to small deviations of the actual coordination
geometry of Er3+ from the C3v
point group symmetry that was assumed in the calculation. Intensities
are analyzed on the level of multiplet-to-multiplet transitions using
the Judd-Ofelt formalism.