Abstract of Publication No. 339
 Ralph Schenker, Høgni Weihe and Hans U. Güdel
Ralph Schenker, Høgni Weihe and Hans U. Güdel
Exchange Interactions Derived from Electron Transfer Processes
in [Cr2(OH)3(tmtame)2](NO3)3
Inorg. Chem. 38, 5593-5601 (1999)
![]()
![]()
Abstract:
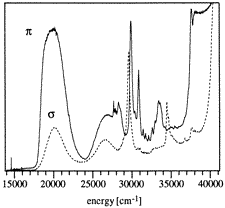
From polarized optical absorption and emission spectra of
[Cr2(OH)3(tmtame)2](NO3)3
(tmtame = N,N',N'
'-trimethyl-1,1,1-tris(aminomethyl)ethane) in the visible and near UV, the
exchange splittings of the 4A24A2
ground as well as the 2E4A2 and
2T14A2 singly and
2E2E, 2E2T1,
2T12T1,
2E2T2, and
2T12T2 doubly excited states of the
ground electron configuration are determined, the latter corresponding to
simultaneous pair excitations by a single photon. The bulk of intensity in the
region of these doubly excited states is found to be vibronically induced by an
electric-dipole exchange mechanism. From single-crystal and glass absorption
spectra a ferromagnetic energy ordering of the lowest energy ligand-to-metal
charge transfer (LMCT) states is derived, whereas the ground state is
antiferromagnetically split. The observed splittings are rationalized using a
model based on a valence bond approach (VBCI), where the exchange interactions
are derived from configuration interaction of LMCT and metal-to-metal charge
transfer (MMCT) electron configurations with the ground configuration. The
splittings are well reproduced by this model over a range of about 40000
cm–1. Trigonal orbital exchange parameters
Ja and Je are derived, revealing that the
direct pathway along the Cr-Cr axis is the dominant one. This gives rise to a
double exchange situation in the LMCT configuration, leading to the observed
ferromagnetic energy ordering of LMCT levels. Magnetostructural correlations are
established from a comparison of the title compound with the similar complex
[Cr2(OH)3(tmtacn)2](ClO4)3
(tmtacn = 1,4,7-trimethyl-1,4,7-triazacyclononane).