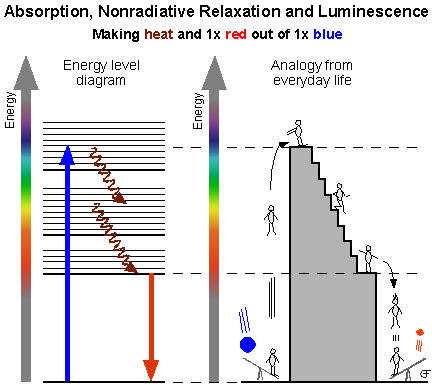
The
spontaneous emission of light upon electronic excitation is
called photoluminescence.
Luminescence is a rare phenomenon among inorganic compounds.
This is due to the predominance of nonradiative relaxation
processes. An electronic excitation of a complex or a metal
center in a crystal usually ends up as vibrational energy and
eventually as heat. In those cases where spontaneous light
emission does occur, its spectral and temporal characteristics
carry a lot of important information about the metastable
emitting state and its relation to the ground state.
Luminescence spectroscopy is thus a valuable tool to explore
these properties. By studying the luminescence properties we can
gain insight not only into the light emission process itself,
but also into the competing nonradiative photophysical and
photochemical processes.
Idea
for picture: Ralph Schenker
Text adapted from: Thomas C. Brunold and Hans. U. Güdel,
"Luminescence Spectroscopy", in "Inorganic
Electronic Structure and Spectroscopy",
E. I. Solomon, A. B. P. Lever (eds.), Wiley, New York, 259-306
(1999)